Levels of IRB Review
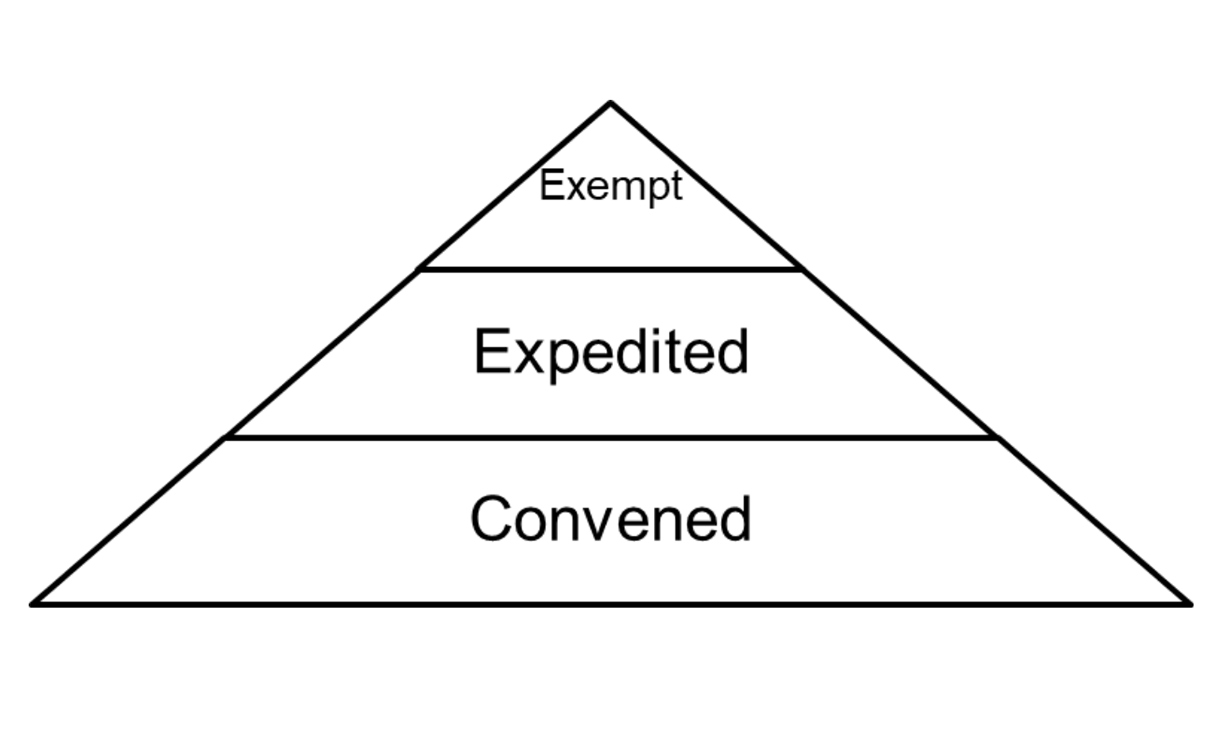
The three levels of IRB review are explained here. Please note that the IRB does not expect researchers to be experts in levels of review and categories of exempt and expedited review. Final determination of level of review and review categories resides with the IRB’s expertise.
-
Exempt / Limited Review
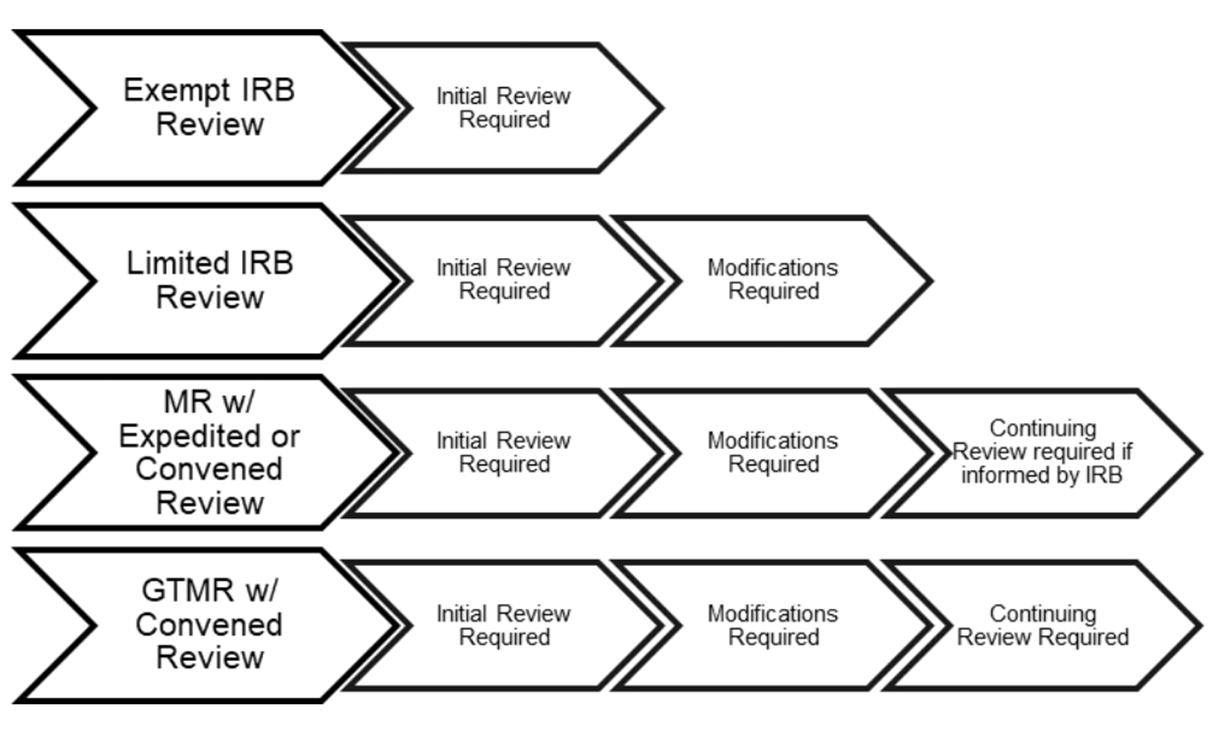
“Exempt” refers to categories of minimal risk human research outlined in the regulations. Exempt does not mean that the research doesn’t have to be submitted to the IRB. “Limited Review” refers to select categories of exempt research under the Revised Common Rule that require ongoing modification submissions to the IRB.
Exempt and Limited Review Categories -
Expedited Review
Projects not eligible for an exempt or limited review may be eligible for an expedited review. “Expedited” refers to categories of minimal risk research outlined in the regulations that require ongoing submissions to the IRB. In general, research may qualify for expedited review if 1) it falls into one of the expedited categories, 2) the risks are minimal, and 3) it does not include sensitive populations or topics.
Expedited Review Categories -
Convened Board Review
Projects not eligible for an exempt or expedited review undergo review by a convened board, even if the study may be minimal risk. The Penn IRB office supports 7 biomedical committees, 1 social behavioral committee, and 1 executive committee. Each committee is supported by a Chair, IRB Director-level regulatory representative, Administrator, and Coordinator.